|
To date the earliest evidence of camelid domestication comes from archaeological sites located between 4,000 and 4,900 m (13,120- to 16,072- foot) elevation, in the puna ecosystem of the Peruvian Andes. Both guanaco (Lama glama cacsilensis) and vicuña (Vicugna vicugna mensalis) have inhabited this tundra environment for approximately 12,000 years and, together with the huemul deer Hippocamelus antisensis (d’Orbigny 1834), were the primary prey of early human hunters. Faunal materials from archaeological sites (Wing 1986; Wheeler 1984, 1986; Wheeler et al. 1976; Moore 1988, 1989) indicate that during the earliest occupation of this zone 12,000 to 7,500 years ago, approximately equal numbers of camelids and deer were hunted, while during later periods the frequency of camelid remains increased dramatically, suggesting a shift to the utilization of domestic animals. Archaeozoological data from one of these sites, Telarmachay Rockshelter, have produced the most extensive evidence concerning this shift to date (Wheeler 1984,1986).
Located 170 km (1,054 miles) northeast of Lima, Peru (11°11’S latitude and 75°52’W longitude) at 4,420 m (14,498 feet) above sea level, Telarmachay is situated near the absolute upper limits of crop growth potential. Mean annual temperature is 4.8°C (40.6 F), with an average daily variation of greater that 20°C (68°F) and frost occurring 330 nights of the year. Annual precipitation averages from 500 to 1,000 mm (20 to 40 inches) and is normally restricted to the months from November to March, although the timing is irregular and unpredictable, and extended periods of drought occur. No agriculture is practiced in the area today, and grazing ungulates represent the most reliable food resource. This is due to their mobility during periods of drought and their ability to convert the dry ligneous puna grasses into a source of stored protein that can be utilized for human consumption. Palaeoclimatoligical data indicate that no significant climatic changes have taken place in this area over the last 10,000 years (Van der Hammen and Noldus 1986).
Five seasons of excavation at Telarmachay Rockshelter (Lavallee et al. 1986) revealed a 8,200-year-long occupational sequence and recovered more than 1 metric ton (1.1 ton) of animal bones from the preceramic levels. Archaeozoolocal analysis of these materials produced evidence of a shift from generalized hunting of guanaco, vicuña, and huemul deer 9,000 to 7,200 years ago, to specialized hunting of guanaco and vicuña approximately 7,200 to 6,000 years ago, then to control of early domestic alpacas and llama to 6,000 to 5,500 years ago, and, finally, to the establishment of a predominately herding economy beginning 5,500 years ago (Wheeler 1986, unpublished data). It has not been possible to determine if these shifts were associated with body size reduction, as has been documented for other domestic ungulates, because species-specific characters for separating postcranial bones are lacking. Instead determination of early camelid domestication at Telarmachay is based upon an increase in the frequency of both camelid and neonatal camelid remains, together with changes in dental morphology. During the preceramic period, 9,000 to 1,900 years ago, camelid remains gradually increased from 64.7 percent to 88.6 percent of the faunal assemblage, while deer remains diminished from 34.2 percent to 9.2 percent of the total (Wheeler 1985). This shift was not caused by decreased availability of deer in the zone, but rather by a change in animal utilization patterns from generalized to specialized hunting and eventual domestication of the camelids.
Between 9,000 and 6,000 years ago, camelid remains increased from 64.7 percent to 81.7 percent of the total faunal sample, with just over one-third (35.3 percent to 37.1 percent) of the bones coming from fetal or neonatal animals (Wheeler 1986). These figures are consistent with a hunting economy because between 35 percent and 40 percent of animals in contemporary guanaco and vicuña populations fall within this category (Franklin 1978, personal communication). Thus, the ever-greater dependence upon camelids in the diet during this period suggests increasing specialization in guanaco and vicuña hunting.
Around 6,000 years ago, however, the frequency of fetal and neonatal camelids increased markedly to 56.8 percent and continued to rise until it reached 73.0 percent of all camelid remains in the deposits dated to 3,800 years ago (Wheeler 1986). These figures suggest either the development of specialized hunting of neonates, an economically unviable strategy or the appearance of other mortality-inducing factors in the environment. They far exceed expected frequencies for both the fetal/neonatal age group and the natural (that is, no human hunting) mortality rates of 4.5 (Raedeke 1979, 199) to 30 percent (Franklin, 1978, 42) that have been recorded for the guanaco and vicuña, but closely correspond with mortality rates experienced by llama and alpaca breeders today.
At present, up to 70 percent of each year’s young may be lost before two months of age owing, in part, to failure of passive immune transfer (Garmendia et al. 1987) with resulting mortality from Clostridium perfringens Type A enterotoxemia and other pathogens (Leguìa 1991; Ramìrez 1991). The epizootic nature of enterotoxemia is to some extent controlled by climatic conditions that permit sporulation of the bacteria, as well as by the presence of a critical number of captive or domestic animals. In the Andes, outbreaks of enterotoxemia are associated with unsanitary corralling practices during the wet-season birth period. Similar epidemics are not known to occur in the wild camelids.
Although it is not always possible to distinguish between the bones of a terminal eleven-and-one-half-month-old fetal camelid and those of a neonate, tooth wear studies indicate that the majority of Telarmachay specimens dating from 6,000 to 3,800 years ago were neonatal, whereas those from the earlier levels were primarily fetal, presumably taken in utero through the hunting of pregnant females (Wheeler 1986). This shift from predominantly fetal to neonatal remains coincides with the significant increase in frequency of fetal/neonatal remains described above and permits the hypotheses that mortality induced by disease rather that by intentional butchery was the cause. Additional support for this interpretation comes from the study of bone distribution across the 6,000- to 3,800-year-old living floors, with indicates that newborn camelids were brought into the shelter whole and processed for consumption. The resultant pattern is very similar to that created by contemporary traditional herders who utilize dead llama and alpaca neonates for food. Meat produced by the often massive die-off of camelid neonates does not now, and apparently did not then, go to waste.
Identification of the species that was brought under domestication at Telarmachay is based upon incisor morphology. Prior to domestication (9,000 to 6,000 b.p. [before the present]) it is estimated that nine vicuña were hunted for every guanaco based on incisor type and frequency. Vicuñas have rootless hypselodont parallel-sided permanent incisors with enamel covering the entire labial surface only (Miller 1924; Wheeler 1982, 1991). Guanacos have rooted deciduous and permanent spatulate incisors with an enamel-covered crown (Miller 1924). By 6,000 b.p.., however, the remains of permanent incisors with the same morphology as deciduous vicuña incisors appear in the Telarmachay deposits (Wheeler 1982, 1991, unpublished date). These permanent teethe match the dentition of many extant Peruvian alpacas in which both the deciduous and permanent incisors are root forming and parallel sided, with enamel covering only the upper labial surface (Wheeler 1991, unpublished data). Although contemporary alpacas with spatulate llama incisors have been reported by Kent (1992), it is unclear if these are hybrids. The evidence from Telarmachay suggests an ancestral relationship, which may explain the apparent retention of juvenile vicuña dental traits in the adult alpaca. It cannot be determined if animals with llama-type incisors also appeared in the 6,000 b.p. deposits, since these are indistinguishable from guanaco incisors, but the presence of both large and small neonates suggests that this may have been the case.
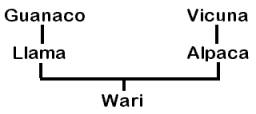
In contrast to the data from Telarmachay and other Andean archaeological sites that indicate that the llama is descended from the guanaco and the alpaca from the vicuña (Figure 1A), other researchers have come to different conclusions about their ancestry based on the study of living animals. In 1775, Frisch attributed the origin of the llama to the guanaco and the alpaca to the vicuña, an opinion subsequently supported by Ledger (1860), Darwin (1868), Antonius (1922), Faige (1929), Krumbiegel (1944, 1952, Steinbacher (1953), Frechkop (1955), Capurro and Silva (1960), Akimushkin (1971), and Semorile, Crisci, and Vidal-Rioja (in press).
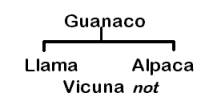
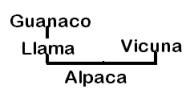
Other authors have concluded that both domestic camelids descend from the guanaco, and the vicuña was never domesticated (Figure 1B) (Thomas 1891; Peterson 1904; Hilzheimer 1913; Lönnberg 1913; Brehm 1916; Cook 1925; Weber 1928; Herre 1952, 1953, 1976, 1982; Röhrs 1957; Fallet 1961; Zeuner 1963; Herre and Thiede 1965; Herre and Röhrs 1973; Bates 1975; Pires-Ferreira 1981/82; Kleinschmidt etal. 1986; Kruska 1982; Jürgens et al. 1988; and Piccinini et al. 1990). In the 1030’s Lòpez Aranguren (1930) and Cabrera (1932) suggested that llama and alpaca evolved from presently extinct wild precursors, based on the discovery fo 2 Myr [million years ago] Plio-Pleistocene L. glama, L. pacos, L. guanicoe, and V vicugna fossils in Argentina, and that the guanaco and vicuña were never domesticated. This position is no longer considered a possible alternative. Finally, Hemmer (1975, 1983, 1990) attributed llama ancestry to the guanaco but has deduced on the basis of shared morphological and behavioral traits that the alpaca originated from hybridization between the llama and vicuña (Figure 1C).
Conclusions about llama and alpaca ancestry have, in large part, been based upon morphological changes produced by the domestication process. During the 1950’s Herre and Röhrs (Herre 1952, 1953, 1976; Herre and Röhrs 1973; Röhrs 1957) examined alterations in the mesotympanal area of the skull, related to a decrease in llama and alpaca hearing acuity, and reported an overall reduction in cranial capacity of both domestic species relative to the guanaco.
In contrast, they found the vicuña cranium to be the smallest of all living Lamini and , based on the premise that domestic animals are smaller than their ancestors, concluded that this species was never brought under human control. Herre and Röhrs consider the llama and alpaca to be “races of the same domestic species bred for different purposes” (Herre 1976,26). Research on the relationship of brain size relative to body size by Kruska (1982) also found the vicuña to be smaller than the alpaca and llama, which in turn were smaller than the guanaco, suggesting that the latter is the only ancestral form. Nevertheless, papers by Jerison (1971) and Hemmer (1990) report the ratio of alpaca brain size to body size to be smaller than in the vicuña, permitting a different conclusion about origins of the domestic forms. These contradictory data on size reduction are almost certainly a product of sampling, as neither subspecific variation in the wild forms nor the possibility of hybridization between the domestic animals were considered in any of the studies. Based on the study of pelage characteristics (skin thickness, follicle structure, secondary/primary ration, fiber length and diameter, coloration) in living camelids, Fallet (1961) found the llama to be an intermediate evolutionary stage between the wild guanaco and the specialized fiber-producing alpaca, and concluded that the absence of transitional characteristics between vicuña and alpaca fleeces eliminates the former from consideration as an ancestral form. This deduction is in part, based on the assumption that llamas have been selected exclusively for use as pack animas while alpaca have been bred for fiber production. Nevertheless, new date on preconquest llama and alpaca breeds in Peru have revealed the prior existence of a fine-fiber-producing llama, as well as an extra-fine-fiber alpaca that is transitional between the vicuña and a second pre-Hispanic fine-fiber alpaca breed (Wheeler, Russel, and Stanley 1992; Wheeler, Russel, and Redden, submitted).
Research on camelid behavior has produced contradictory hypotheses concerning llama and alpaca origins, Krumbiegel (1944, 1952) and Steinbacher (1953) argue that the alpaca is the domestic vicuña based on unique shared behavioral traits that are said to differ from those observed in the guanaco and llama. Hemmer, on the other hand, concludes that while some alpaca behavior patterns match those of the vicuña, others are intermediate between those of vicuña and guanaco, suggesting that “The alpaca is a mixture of both line, [produced] by crossbreeding of captured vicuñas with the only initially available domestic animal, the llama” (1900, 63). It has also been suggested that the vicuña was never domesticated because it is more territorial than the guanaco (Franklin 1974). Nevertheless, this assumption is open to question because it is based upon study of guanacos located at the southernmost extreme of their range, where seasonal migration in response to server climatic changes is essential for survival (Franklin 1982, 1983). Farther to the north, where vicuña and guanaco ranges overlap and llama and alpaca domestication occurred (Wheeler 1984), a more benign climate and a constant food supply permit the characteristic sedentary social organization of the vicuña (Franklin 1982, 1983). Although data concerning behavior of the guanaco in this region are lacking, it is possible that the limited sedentary territorial organization observed in some Patagonian groups plays a more important role in these less-extreme climatic conditions.
Analysis of hemoglobin amino acid sequences in vicuña, alpaca, llama and guanaco from the Hannover Zoo, Germany, led Kleinschmidt et al. (1986), Jürgens et al, (1988), and Piccinini et al. (1990) to the conclusion that the vicuña was never domesticated. However, earlier research on blood and muscle samples from llama, alpaca, vicuña, guanaco, and alpaca x vicuña hybrids at Santiago Zoo (Cappuro and Silva 1960) indicated a llama-guanaco and alpaca- vicuña subdivision, as have more recent data from ribosomal genes (Semorile et al., in press). Other researchers utilizing immunological, electrophoretic analysis, and protein sequencings have found it impossible to draw conclusions about llama and alpaca ancestry (Miller, Hollander, and Franklin 1985; Penedo et al. 1988). Cytogenetic studies (Capanna and Civitelli 1965; Taylor et al. 1968; Larramendy et al. 1984; Gentz and Yates 1986) indicate that all four species of the South American Camelidae have the same 2n = 74 karyotype, but information on molecular biology is limited. Vidal-Rioja et al. (1987) and Saluda-Gorgul, Jaworski and Greger (1990) have examined satellite DNA, and research analyzing the full mitochondrial cytochrome b gene sequence in all six Camelidae has documented hybridization among the domestic South American camelids (Stanley et al. 1994). Recent studies of the fiber from mummified ninth- and tenth- century llamas and alpacas suggests that post conquest hybridization has modified the genetic makeup of living populations (Wheeler et al 1992), a fact that may well explain the diversity of conclusions about their ancestry.
The llama is the largest of the domestic South American camelids and resembles its ancestor in almost all aspects of morphology and behavior.
Like the guanaco, the llama has adapted to a wide range of environments (Figure 2C). After domestication in the Peruvian puna between 7,000 and 6,000 year ago (Wheeler 1984, 1991; Wing 1977, 1986), the llama was moved to the lower-elevation inter-Andean valleys and into northern Chile, where their remains have been found in archaeological sited dated to 3,800 years ago (Wing 1986; Hesse 1982; Dransart 1991a). Some 2,300 years later they were being bred on the north coast of Peru (Shimada and Shimada 1985) and in Ecuador (Wing 1986; Stahl 1988; Miller and Gill 1990).
Although it is often assumed that the Lake Titacaca region was also a center of llama domestication, relevant data are lacking from early archaeological sites in Bolivia (Browman 1989). In northwestern Argentina, a single cranium of L. glama has been dated to 3400 b.p., with stronger evidence for herding at 1450 b.p. (Yacobaccio and Madero 1992; Reigadas 1992), and it is thought that domestication may have occurred independently in both this region (ibid.) and northern Chile (Hesse 1982).
Shortly thereafter, 900-1000 b.p., evidence of llama rearing has been recovered at sites located in the cloud forest on the eastern slope of the central Andes, as well as in the dry Osmore drainage of south coastal Peru (Wheeler 1991, in press). Under Incan rule (1479-1532) llama distribution reached its farthest expansion as pack trains accompanied the royal armies to southern Colombia and central Chile. It is impossible to estimate the size of this preconquest llama population, but it clearly must have exceeded present numbers, for early Spanish administrative documents record the virtual disappearance of these animals within a century of contact (Flores Ochoa 1977). In recent years, the llama population has remained relatively stable, totaling 3,776,793 in 1991 (Wheeler 1991).
Because Andean civilization was nonliterate, knowledge of pre-Spanish llama- and alpaca-herding practices must be reconstructed from archaeological remains. The recent discovery of 900- to 1,000-year old naturally desiccated llama s and alpaca at El Yaral, an archaeological site in the Moquegua valley of southern Peru (Rice 1993), has provided a first view of preconquest Inca Chiribaya culture, these animals had been sacrificed by a blow between the ears and immediately buried beneath house floors, where they became naturally mummified from the extreme aridity of the environment.
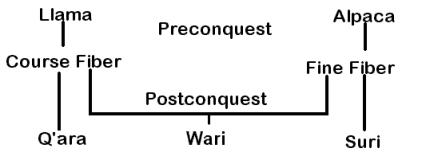
Research on the physical appearance of the El Yaral llamas, as well as analysis of skin and fiber samples taken at eleven different locations across the body, revealed the possible existence of both a fine-fiber and a coarse-fiber breed (Wheeler et al. 1992; Wheeler et al. submitted ) (Figure 3).
The Spanish conquest had a disastrous effect on both llama and alpaca population. Massive mortality accompanied the displacement of alpaca herds from the coast, inter-Andean valleys and most of the puna, as introduced stock rearing practices pushed the survivors into the marginal, extreme high-elevation pastures where they are found today (Flores Ochoa 1982). At present, alpaca distribution extends from approximately 8 ° S latitude, in the vicinity of Lake Poopo, Bolivia, with small population located farther to the south in northern Chile and northwestern Argentina (Figure 2D).
Today, 75 percent of all alpacas, paqocha in Quechua (Flores Ochoa 1988) and allpachu in Aymara (Dransart 1991b), are held by traditional herders (Novoa 1989). Two alpaca phenotypes, known in the literature by their Quechua names as “suri and huacaya” or wakaya, are recognized but these do not breed ture. The suri has long straight fibers, organized in waves that fall to each side of the body in much the same manner as a Lincoln sheep, while the huacaya has shorter, crimped fibers that give it a spongy appearance similar to that of a Corriedale sheep. Occasionally animals with intermediate wool characteristics are seen, and these have been named “chili” by Cardozo (1954). Crosses between huacaya and huacaya produce a certain percentage of suri offspring, and crosses of suri with suri produce some huacaya offspring. Although no artificial selection is made, an estimated 90 percent of all alpacas are huacayas (Novoa 1989). The suri is not known among the Aymara herders of Chile, who refer to their huacayas simply as allpachu or alpacas (Dransart 1991b). The fleece of both phenotypes varies from white to black and brown, passing through all intermediate shades, with a greater tendency to uniform coloration than in the llama. Alpacas with wild vicuña coloration occur.
In comparison to the preconquest El Yaral alpacas, contemporary Andean huacaya and suri fleeces average 31.2+3.8 m m (Carpio 1991) and 26.8+6.0 m m (Von Bergen 1963) respectively, are coarser, may have a tendency to hairiness, and are of uneven quality. Some coats containing up to 40 percent hair have been reported for both living varieties, and considerable variation is reported in published statistics on fiber diameter. The origin of this degeneration almost certainly lies in the Spanish conquest, but a breakdown in controlled breeding between the fine and extra-fine El Yaral breed would not alone account for the variation observed today.
The most probable cause of coarsening and hairiness in both huacayas and suris would be through hybridization with the coarse-fiber llama breed, a not improbable scenario amid the chaos and destruction of the conquest. Clearly, however, such a process would not have affected only the alpaca gene pool. The El Yaral mummies indicate the possibility that extensive crossbreeding between alpacas and llamas may have occurred since the Spanish conquest and has played a much more important role in the formation of today’s livestock than has been realized (Figure 3).
HYBRIDIZATION
The guanaco, vicuña, llama, and alpaca all possess the same 2n = 74 karyotype (Capanna and Civatelli 1965; Taylor et al. 1968) and can, under human influence, produce fertile hybrids (Gray 1954). Preliminary research on the cytochrome b gene sequence has found no evidence of hybridization between guanaco and vicuña (Stanley et al. 1994). Because this study included samples from the northern population of both genera, the region where they are sympatric in parts of their range, the findings may possibly support Miller’s (1924) creation of the genus Vicugna.
Traditional herders recognize the existence of llama and alpaca crosses. These are referred to by the generic terms wari in Quechua (Flores Ochoa 1988) and wik’ñna in Aymara (Dransart, 1991b). These hybrids are classified as llamawari or llama-like, and paqowari or alpaca-like, by Quechua speakers (Flores Ochoa 1977). Aymara speaking herders use waritu and wayki for llama and alpaca phenotype hybrids, as well as the generic term wakayu for any llama/alpaca offspring (Dransart 1991b). First-generation crosses are easily recognized, but it is not always possible to identify hybrid animals based upon phenotype alone because it is likely that hybridization has been an ongoing process since the time of the Spanish conquest. The extent to which contemporary llama and alpaca populations have been affected by this process has not been determined, but comparison with preconquest animals suggest that it has been extensive and that breeds of fine-fiber-producing llama and alpaca have likely disappeared in the process (Wheeler et al. 1992). Hybridization has been confirmed through DNA analysis (Stanley et al. 1994) (Figure 3).
Crosses between the wild and domestic South American camelids produce fertile offspring but do not normally occur in nature. The pacovicuña, or alpaca/vicuna hybrid, has received considerable attention for its potential as a fine-fiber producer. Carpio etal. (1990) report fiber diameters ranging from 13.3 to 17.3 m m for five first-generation crosses, but this is said to rapidly increase in subsequent generations. The pacovicuña phenotype closely resembles that of the vicuña, although it is slightly larger and less gracile than its wild progenitor. Research on the fixation of phenotypic traits from generation to generation of alpaca/vicuña hybrids is lacking, and much remains to be done before its potential as a fine-fiber producer can be evaluated.
The possibility that feral llamas and alpacas exist and might have crossed with wild camelids has not been fully explored. In 1534, Xerez observed that domestic llamas were sometimes so numerous some escaped to the wild, and in 1555 Zarate recorded that once each year some llamas were released into the wild as an offering to the gods (Murra 1978). It is unclear, however, if feral populations existed at that time. The current consensus of opinion in the central Andean region is that no such populations exist today. Even so, MacDonagh (1940) has described a group of guanaco and llama hybrids living in a feral state in the province of Cordoba, Argentine. These animals were the product of natural crosses and generally exhibited the guanaco phenotype, although some had white blotches on the head and upper part of the neck, and others were almost entirely white. No observations of changes in body size and fiber quality were recorded. The behavior of these feral hybrids was considered to be virtually identical with that of the guanaco, and they lived and reproduced without problem.
THE FUTURE
The present status of the South American camelids is the product of a largely unknown past. To name but two historic factors, the potential influence of genetic bottlenecks and/or hybridization in the formation of contemporary guanaco, vicuña, llama, and alpaca population have never been fully investigated, although there is evidence to suggest that they may have played an important role. The most basic questions concering genetic variation and the systematic classification of presumed guanaco and vicuña subspecies, as well as llama and alpaca breeds, remain to be answered, although such information is essential for ensuring their future. In the case of the wild camelids, we need to be sure that we are protecting all genetic variants of each species, and not just increasing the numbers of potentially genetically impoverished subgroups. In light of the increased movement of both wild and domestic camelids throughout the Andes and the beginning of exportation in 1983, there is an urgent need to identify relict populations of genetically pure pre-Columbian llama and alpaca breeds in order to ensure both their preservation and the possibility of a return to high-quality fine-fiber production.
~~~~~~~~~~
Mitochondrial genes have been much used in the study of evolution because they evolve rapidly, but since they only contain information from the female line they are of limited use in cases of hybridization. In an initial attempt to resolve the question of alpaca and llama origins, Helen Stanley, Miranda Kadwell and Jane Wheeler sequenced part of the mitochondrial cytochrome b gene (Proceedings of the Royal Society London B 256:1-6.1994). Their results confirmed that Lama and Vicugna are valid genera which separated 3-2 Million years ago, but also produced evidence of considerable hybridization in the domestic forms which made it impossible to determine their origins using mitochondrial data alone. For this reason they turned to the study of nuclear (maternally and paternally inherited) DNA and in 2001 Miranda Kadwell, Matilde Fernandez, Helen Stanley, Ricardo Baldi, Jane Wheeler, Raul Rosadio and Michael Bruford published the article “Genetic analyses reveals the wild ancestors of the llama and alpaca” in the Proceedings of the Royal Society London B 268:2575-2584. Utilizing a large sample set collected throughout the geographic range of the four South American camelids (n=771) the authors examined both nuclear (4 microsatellites) and mitochondrial (cytochrome b) DNA sequences. Their results indicate that the alpaca descends from the vicuñ a and the llama from the guanaco; that the correct classification of these forms is Vicugna pacos and Lama glama respectively, and that such extensive hybridization has occurred that only 20% of alpacas and 60% of llamas are genetically pure.
The implications of these data are very important for the way in which these genetic resources should be managed in future. During the last 20-25 years large-scale hybridization between llama and alpacas has been carried out in the Andes. Specifically, male alpacas have been bred to female llamas to increase the population of animals producing higher priced “alpaca” fiber, and male llamas have been bred to female alpacas to obtain greater fleece weights and thus, increased income. With sale price traditionally determined by weight, and no consideration given to fineness, the quality of alpaca fiber has decreased markedly over the past 25 year. Indigenous Quechua and Aymara speaking herders subdivide the hybrids into llamawari or waritu (llama-like) and pacowari or wayki (alpaca-like) respectively, depending upon physical appearance. The F1 offspring are fertile, tend to be intermediate in size and can be back-crossed to either parental type producing fertile young. Further, recent intensive selection for white fleece may also have involved bi-directional hybridization. This situation may well explain the long standing debate concerning alpaca and llama origins, since it is likely that many specimens used in previous studies were hybrids.
With such a high incidence of hybridization documented for the Andean Alpaca and llama populations, it is clear that DNA testing is required in order to determine which animals are hybrids and which are pure. The Peruvian research instutute CONOPA – Coordinadora de Investigacion y Desarrallo de Camelidos Sudamericanos. jwheeler@conopa.org, founded by Jane Wheeler, Eugenio Artaza-Llona and Raul Rosadio continues research on the topic and offers testing for genetic purity (patent pending). Recently, with financial support from World Bank/Incagro Peru, CONOPA conducted an evaluation of the alpaca population in Canchis Province, Cusco. Using a stratified random sample, 3,000 animals (out of approximately 120,000) were evaluated and 600 tested for genetic purity and fibre characteristics. CONOPA is also actively involved in the establishment of a registry of genetically pure animals and setting-up core herds in order to insure their survival.
Dr. Wheeler holds degrees from American University, Cambridge University, and the University of Michigan, and completed postdoctoral studies at eh University of Paris. She has been involved in broad-based research on South American camelids for more than twenty five years, publishing extensively in English, French and Spanish (4 books, 20 book chapters, more that 40 articles in scientific journals, and miscellaneous popular articles among others) on topics ranging from origin and evolution of domestic alpacas and llamas, to production, breeding and fibre production, as well as vicuña conservation. Her work on camelid genetics began in 1992 while she was a Visiting Scientist at the Macaulay Land Use Research Institute in Scotland and continues to this day. Together with Eugenio Artaza-Llona and Raul Rosadio, she is a founding member of CONOPA, a research and development organization working towards a systemic solution of the problems confronting the Peruvian South American camelid sector. In the area of genetics, she directs CONOPA’s current project portfolio which includes European Community funding for work on sustainable utilization of the vicuña (MACS), Darwin Initiative for the Survival of Species (UK) support for the evaluation of genetic diversity and development of a management plan for Peru’s endangered Andean guanaco populations and a World Bank/Incagro financed project for identification and rescue of the genetically pure alpaca from the threat of extinction.
|

